Heat Shield
Water boils at 212° Fahrenheit (100° Celsius) at standard pressure. If you take a few drops of water and toss them into a frying pan at 212° Fahrenheit or above, therefore, the water will start to boil. The water makes a sizzling sound as it rapidly evaporates and steam shoots out of the pan.
But sometimes, that doesn’t happen. Sometimes, water droplets form in the pan and remain there, not boiling off. They tend to dance around the pan, too, as seen in the video below. As the woman in the video notes, the water is “spinning around like crazy” despite the fact that the pan is hot — certainly hotter than needed for the water to boil.
What’s going on here? The pan is definitely hotter than 212° Fahrenheit, and likely above 350° Fahrenheit (176° Celsius). The water droplets aren’t boiling immediately because the pan is too hot, a phenomenon called the Leidenfrost effect.
Identified in 1754 by a German scientist named Johann Gottlob Leidenfrost, the eponymous result happens because the water — some of it, at least — simply boils too quickly. That part of the water vaporizes and creates a cushion between the rest of the water and the hot metal surface below, as seen in this very helpful diagram courtesy of Wikipedia:
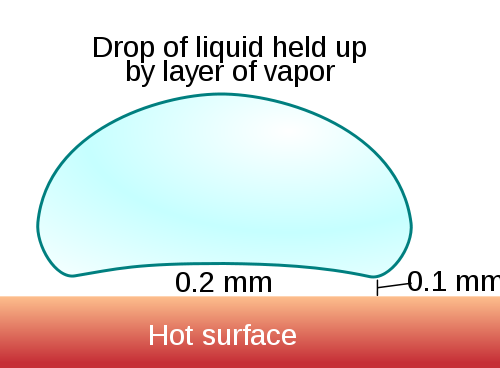
As the water is not in contact with the surface, it therefore doesn’t hit the boiling point as quickly as it would otherwise, and takes much longer to boil (and in general, won’t until the vapor layer dissipates). Further, because the water is sitting on top of the vapor, it tends to dart around the pan in seemingly random directions.
The Leidenfrost effect isn’t limited to drops of water in pans, either. One researcher (and don’t try this at home!) dipped his wet hands in molten lead and came out unscathed, as the water on his hand vaporized, forming a bubble of sorts separating his skin from the superheated metal. And one hobbyist heated a ball of nickel until it was red hot and dropped it in water. The end result, as this video shows, is a crazy mix of sounds as the vapor shell encasing the ball dissipates over fifteen seconds or so.
Bonus Fact: Water vapor isn’t steam. Steam is the gaseous form of water and is invisible, while water vapor is what we see (out of a tea kettle, as Mr. Wizard used by way of example in making this same point) and often call “steam.” We see water vapor because the steam is cooling as it comes in contact with air, or, in the case of the Leidenfrost effect, air and the water above.
From the Archives: Why Our Fingers Wrinkle When Wet.
Related: Want to create some science experiments in your kitchen? Here’s a $10 kit.
